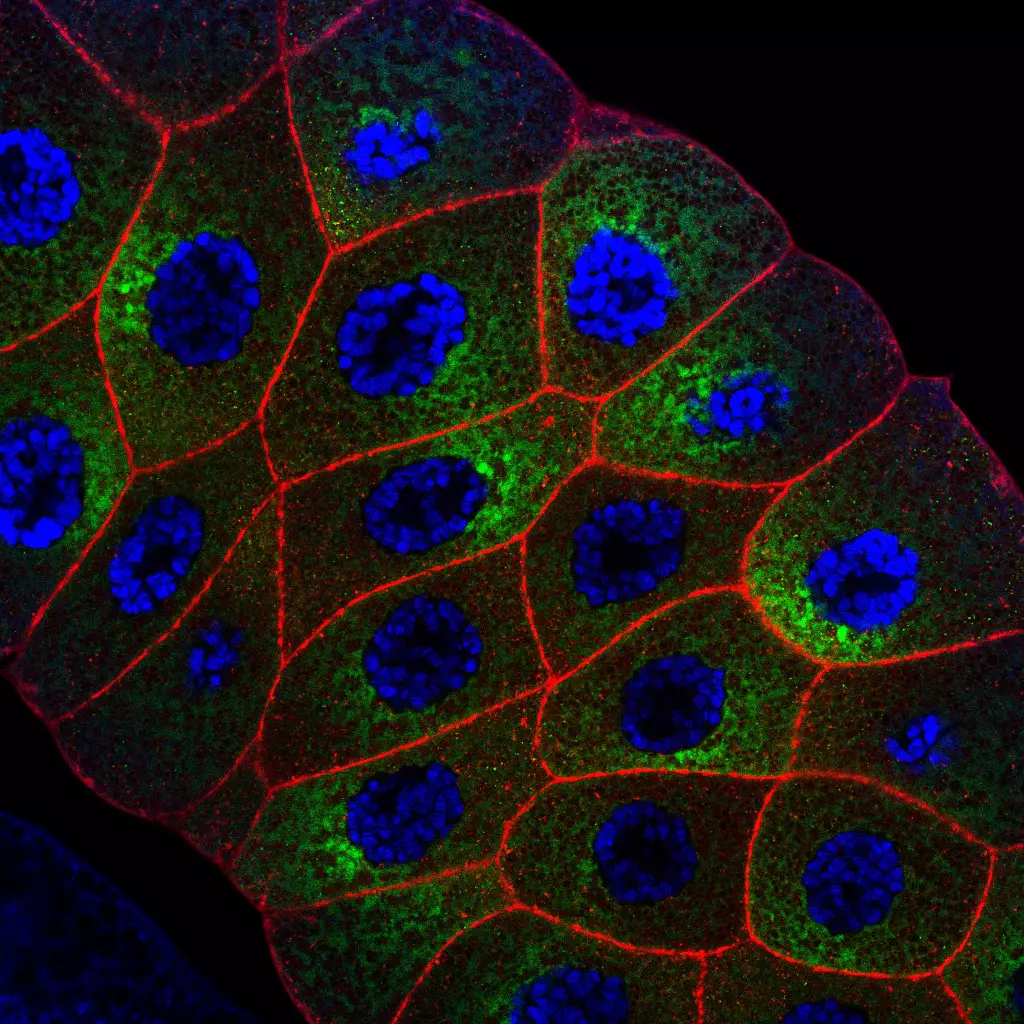
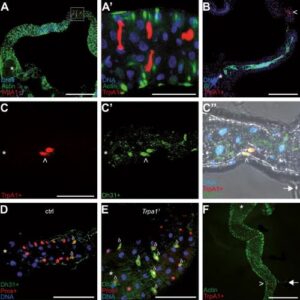
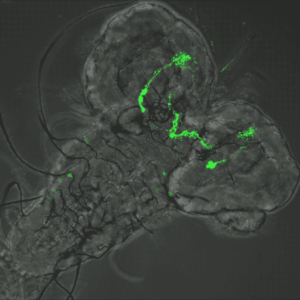
Host Pathogen interaction in the Drosophila model
Our team studies, at the molecular level, the impact that bacteria have on the metabolism and the behavior of their hosts.
Eucaryotes live in diversified ecological niches that, while variable in terms of physical and chemical characteristics, are all colonized by microorganisms such as bacteria, viruses or fungi. It follows that, from the earliest stages of their development until their death, animals interact for better or for worse with these co-inhabitants. For the better, as microbes can positively impact various physiological parameters of the host such as fecundity, longevity, and growth, to name but a few. For worse, since, obviously, some of these microbes can negatively affect the host and can even sometimes be life-threatening for them. To defend themselves, eucaryotes have developed immune strategies to identify surrounding microorganisms and trigger ad hoc responses that eradicate invaders and ensure the integrity and fitness of the host and its progeny. Recent studies highlight the benefits of bringing neurons into the complex host–microbe interaction game. Sensory neurons play a role in identifying microbes and, thus, in distinguishing beneficial ones to live with from other, potentially pathogenic, ones to avoid. In contrast, host neurons can be hijacked by microorganisms and microbe-derived products to ease their proliferation within infected animals. In addition, the nervous system’s perception of a microbial threat may allow the host to modify its behavior to reduce the consequences of the infestation on itself and its offspring.
As neuroscientists and immunologists continue to uncover molecules acting across both systems and genetic interactions between them, it becomes clear that the immune and the neuronal systems share many components, and cooperate at many different levels to allow an animal to live in harmony with its exogenous and endogenous microbes. Using its powerful collection of genetic and genomic tools, we used Drosophila to understand how immune and neuronal mechanisms cooperate to enable flies to protect themselves from pathogenic microbes or sometimes to take advantage of the microorganisms they live with.
Publications
Bacteria-Derived Peptidoglycan Triggers a Noncanonical Nuclear Factor-κB-Dependent Response in Drosophila Gustatory Neurons
Gut-derived peptidoglycan remotely inhibits bacteria dependent activation of SREBP by Drosophila adipocytes
How Bacteria Impact Host Nervous System and Behaviors: Lessons from Flies and Worms
Peptidoglycan-dependent NF-κB activation in a small subset of brain octopaminergic neurons controls female oviposition
Cytosolic and Secreted Peptidoglycan-Degrading Enzymes in Drosophila Respectively Control Local and Systemic Immune Responses to Microbiota
Drosophila’s sensory responses to bacterial peptidoglycan integrates positive and negative signals
Larval microbiota primes the Drosophila adult gustatory response
Bacteria-Derived Peptidoglycan Triggers a Noncanonical Nuclear Factor-κB-Dependent Response in Drosophila Gustatory Neurons
Gut-derived peptidoglycan remotely inhibits bacteria dependent activation of SREBP by Drosophila adipocytes
How Bacteria Impact Host Nervous System and Behaviors: Lessons from Flies and Worms
Gut bacteria-derived peptidoglycan induces a metabolic syndrome-like phenotype via NF-κB-dependent insulin/PI3K signaling reduction in Drosophila renal system
Drosophila Aversive Behavior toward Erwinia carotovora carotovora Is Mediated by Bitter Neurons and Leukokinin
Peptidoglycan-dependent NF-κB activation in a small subset of brain octopaminergic neurons controls female oviposition
Cytosolic and Secreted Peptidoglycan-Degrading Enzymes in Drosophila Respectively Control Local and Systemic Immune Responses to Microbiota
Oligopeptide Transporters of the SLC15 Family Are Dispensable for Peptidoglycan Sensing and Transport in Drosophila.
Drosophila larvae food intake cessation following exposure to Erwinia contaminated media requires odor perception, Trpa1 channel and evf virulence factor
Inhibition of a NF-κB/Diap1 Pathway by PGRP-LF Is Required for Proper Apoptosis during Drosophila Development
Bacteria sensing mechanisms in Drosophila gut: Local and systemic consequences.
Tissue-Specific Regulation of Drosophila NF-x03BA;B Pathway Activation by Peptidoglycan Recognition Protein SC.
Drosophila Microbiota Modulates Host Metabolic Gene Expression via IMD/NF-κB Signaling.
Mutations in the Drosophila ortholog of the vertebrate Golgi pH regulator (GPHR) protein disturb endoplasmic reticulum and Golgi organization and affect systemic growth.
Mecanisms and consequences of bacteria detection by the Drosophila midgut.
The Drosophila inner-membrane protein PMI controls cristae biogenesis and mitochondrial diameter.
Peptidoglycan sensing by the receptor PGRP-LE in the Drosophila gut induces immune responses to infectious bacteria and tolerance to microbiota.
Gut-microbiota interactions in non-mammals: what can we learn from Drosophila?
Peptidoglycan recognition proteins: modulators of the microbiome and inflammation.
Epithelial homeostasis and the underlying molecular mechanisms in the gut of the insect model Drosophila melanogaster.
Toll-8/Tollo negatively regulates antimicrobial response in the Drosophila respiratory epithelium.
Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing.
The Drosophila peptidoglycan-recognition protein LF interacts with peptidoglycan-recognition protein LC to downregulate the Imd pathway.
Polyglutamine Atrophin provokes neurodegeneration in Drosophila by repressing fat.
Inner-membrane proteins PMI/TMEM11 regulate mitochondrial morphogenesis independently of the DRP1/MFN fission/fusion pathways.
Lack of an antibacterial response defect in Drosophila toll-9 mutant.
Drosophila immune response: From systemic antimicrobial peptide production in fat body cells to local defense in the intestinal tract.
Elimination of plasmatocytes by targeted apoptosis reveals their role in multiple aspects of the Drosophila immune response.
Bacterial detection by Drosophila peptidoglycan recognition proteins.
The Drosophila membrane-associated protein PGRP-LF prevents IMD/JNK pathways triggering by blocking PGRP-LC activation.
Crystal structure of Drosophila PGRP-SD suggests binding to DAP-type but not lysine-type peptidoglycan. Molecular Immunology.
Peptidoglycan recognition proteins: pleiotropic sensors and effectors of antimicrobial defences.
Downregulation of the Drosophila Immune Response by Peptidoglycan-Recognition Proteins SC1 and SC2.
Infectious non-self recognition in invertebrates: lessons from Drosophila and other insect models.
Function of the drosophila pattern-recognition receptor PGRP-SD in the detection of Gram-positive bacteria.
Drosophila melanogaster innate immunity: an emerging role for Peptidoglycan Recognition Proteins in bacteria detection.
Toll-dependent and Toll-independent immune responses in Drosophila.
Dual activation of the Drosophila Toll pathway by two Pattern Recognition Receptors.
Silencing of Toll pathway components by direct injection of double-stranded RNA into Drosophila adult flies.
Notch signaling controls lineage specification during Drosophila larval hematopoiesis.
The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein.
Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein.
News
Using ROS, larvae coordinate trapping harmful gut bacteria with a valve, then kill them with antimicrobial peptides.
5 motivated and talented students successfully defended their thesis between September 2022 and January 2023.
Our work demonstrates that by activating the NF-kB pathway, cell wall peptidoglycan produced by these same bacteria, counteracts SREBP activation in adipocytes in a cell-autonomous manner.
7 IBDM teams have received grants from ANR
7 IBDM teams have received grants from the Agence Nationale pour la Recherche (ANR) in 2021. Congratulations to Vincent Bertrand, Harold Cremer, Pascale Durbec, André Le Bivic, Pierre-François
In a recent article published in iScience, Bernard Charroux, Fabrice Daian and Julien Royet analyze the behavior of fruit flies simply put in contact with bacteria potentially pathogenic to them.
Julien Royet’s team uses the genetic tools generated in the fruit fly to dissect the molecular interactions between bacteria and the host’s nervous system.
No jobs opportunities found..